Aromatic organic compounds
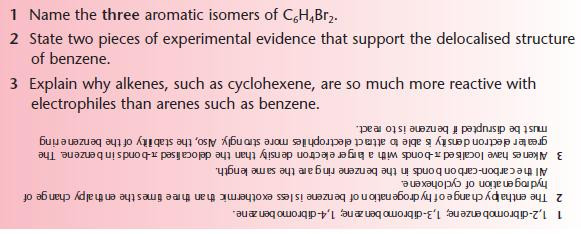
ARENES
After studying this section you should be able to:
- apply rules for naming simple aromatic compounds
- understand the delocalised model of benzene
- explain the resistance to addition of benzene compared with alkenes
Aromatic organic compounds
Image
Image

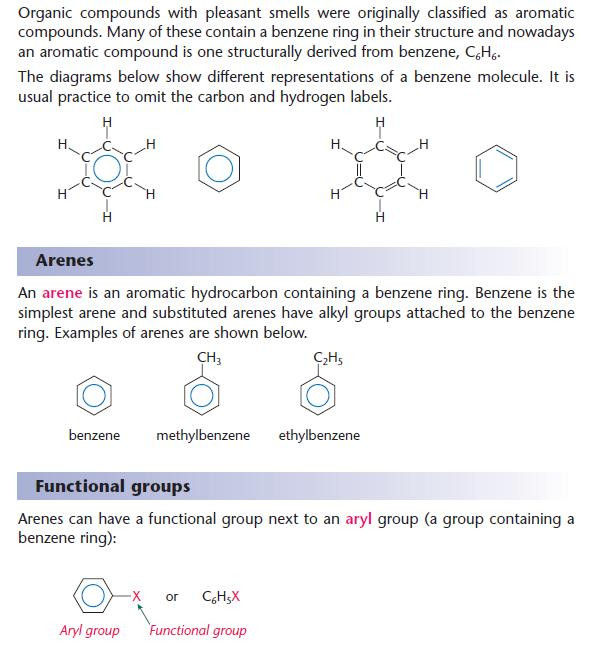
- An aryl group contains a benzene ring.
Naming of aromatic organic compounds
Image
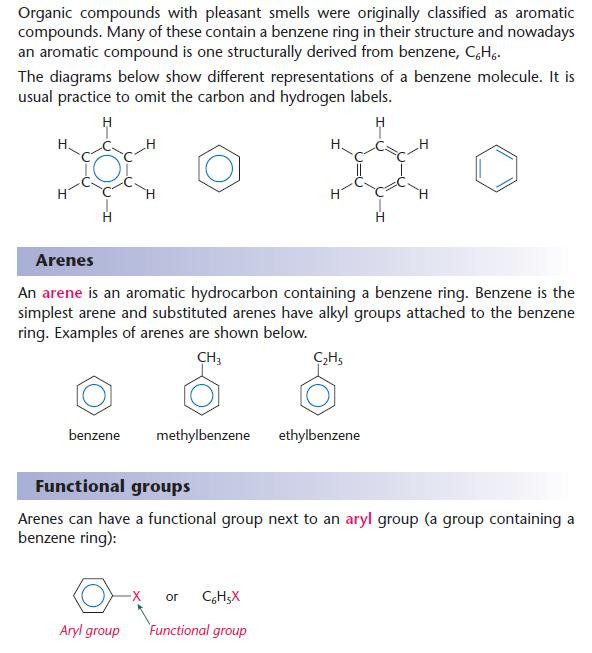
- You should be able to suggest names for simple arenes.
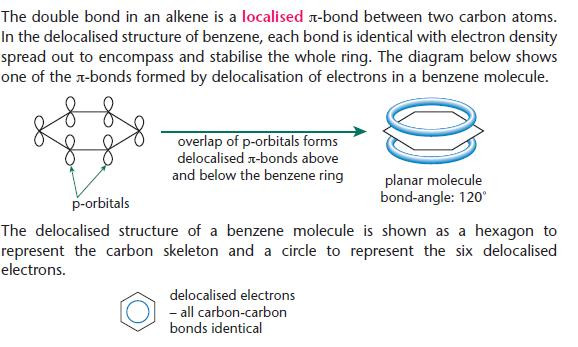
The stability of benzene
Two structures are used to represent benzene:
- the Kekulé structure developed between 1865 and 1872
- the modern delocalised structure developed in the 1930s.
Image
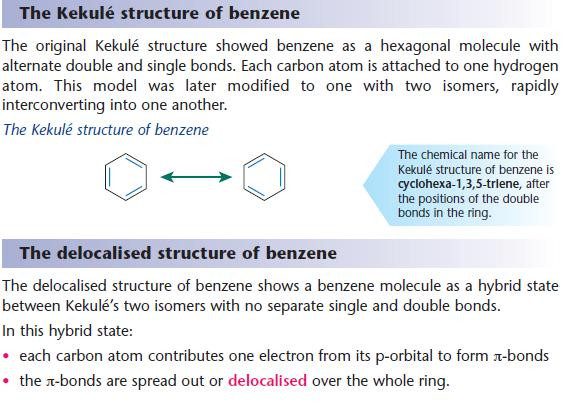
- The Kekulé model of a benzene molecule has alternate double and single bonds making up the ring.
- The delocalised model of a benzene molecule has identical carbon–carbon bonds making up the ring.
Image
- Key point from AS - Alkenes
- This model helps to explain the low reactivity of benzene compared with alkenes.
- Although the Kekulé structure is used for some purposes, the delocalised structure is a better representation of benzene. You will find both representations in books.
The shape of a benzene molecule
Image
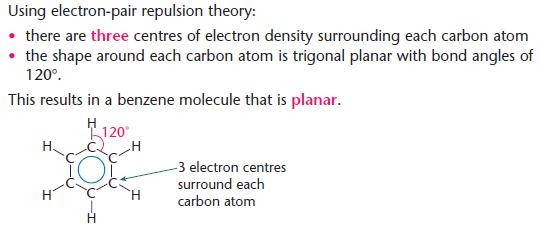
- Key points from AS - Electron-pair repulsion theory
Experimental evidence for delocalisation
Image
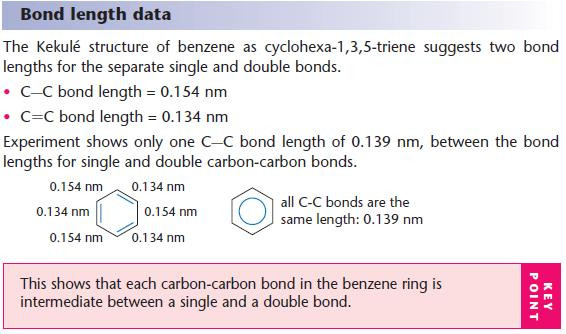
Thermochemical evidence
Image
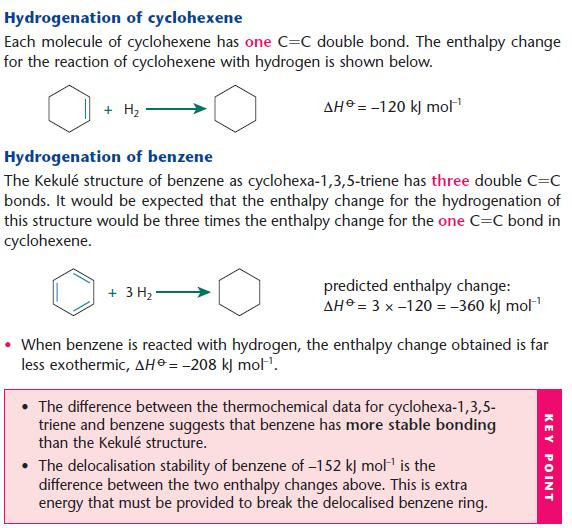
- The stability of benzene can also be demonstrated using thermochemical data for the reactions of bromine with cyclohexene and benzene.
Comparing electrophilic addition to arenes and alkenes
Arenes and alkenes both have π-bonds, but
- arenes have delocalised π-bonds
- alkenes have localised π-bonds
- the delocalised electron density in arenes is less than in alkenes.
Alkenes react with bromine in the dark at room temperature. Using the same conditions with benzene, there is no reaction. The stability of the delocalised system resists addition which would disrupt this stability.
Key points from AS
- Alkenes
- Addition reactions of alkenes
PROGRESS CHECK 1
Image