The Structure of Proteins
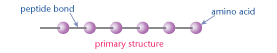
Primary protein structure
This is the linear sequence of amino acids.
Secondary protein structure
Polypeptides become twisted or coiled. These shapes are known as the secondary structure. There are two common secondary structures; the α-helix and the β-pleated sheet.
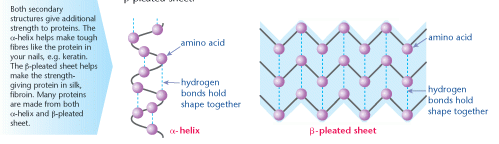
The polypeptides are held in position by hydrogen bonds. In both α-helices and β- pleated sheets the C=O of one amino acid bonds to the H–N of an adjacent amino acid, like this, C = O - - - H–N.
An α-helix is a tight, twisted strand; a β-pleated sheet is where a zig-zag line of amino acids bonds with the next, and so on. This forms a sheet or ribbon shape.
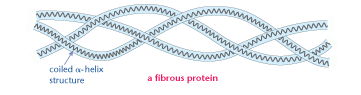
The protein shown, only achieves a secondary structure as the simple αhelix polypeptides do not undergo further folding. This is the structure of a fibrous protein. It is made of three αhelix polypeptides twisted together.
Tertiary protein structure
This is when a polypeptide is folded into a precise shape. The polypeptide is held in ‘bends’ and ‘tucks’ in a permanent shape by a range of bonds including:
- disulphide bridges (sulphur–sulphur bonds)
- hydrogen bonds
- ionic bonds.
Note that the specific contours of proteins have extremely significant roles in life processes.
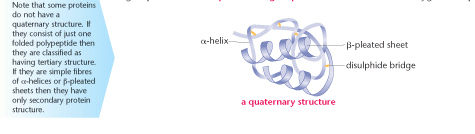
Quaternary protein structure
This is the structure of a globular protein. It is made of an α-helix and a β-pleated sheet. Precise
shapes are formed with specific contours.
Some proteins consist of different polypeptides bonded together to form extremely intricate shapes. A haemoglobin molecule is formed from four separate polypeptide chains. It also has a haem group, which contains iron. This inorganic group is known as a prosthetic group and in this instance aids oxygen transport.
This video explains the strucure of proteins
