Polymers
After studying this section you should be able to:
- describe the characteristics of addition polymerisation
- describe the characteristics of condensation polymerisation in polypeptides and proteins, polyamides and polyesters
- discuss the disposal of polymers
During the study of alkenes in AS Chemistry, you learnt about addition polymers. For A2 Chemistry, addition polymerisation is reviewed and compared with condensation polymerisation.
Monomers and polymers
A polymer is a compound comprising very large molecules that are multiples of simpler chemical units called monomers.
Monomers are small molecules which can combine together to form a single large molecule, called a polymer.

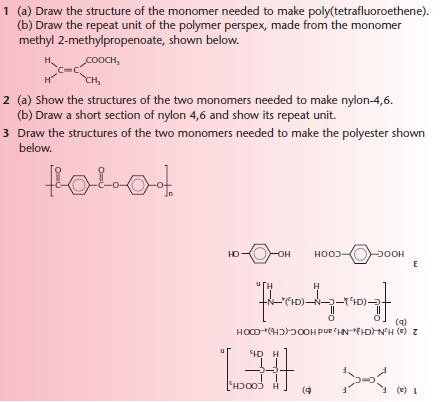
Addition polymerisation
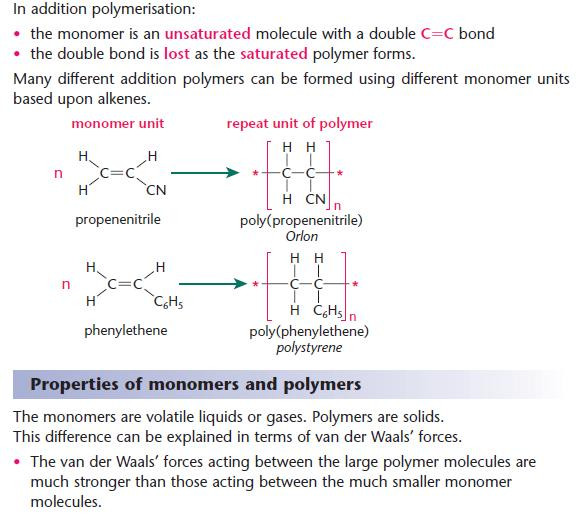
- Key points from AS - Addition polymerisation of alkenes
- Notice that n monomer molecules produce one polymer molecule with n repeat units.
- You should be able to draw a short section of a polymer given the monomer units (and vice versa).
Condensation polymerisation
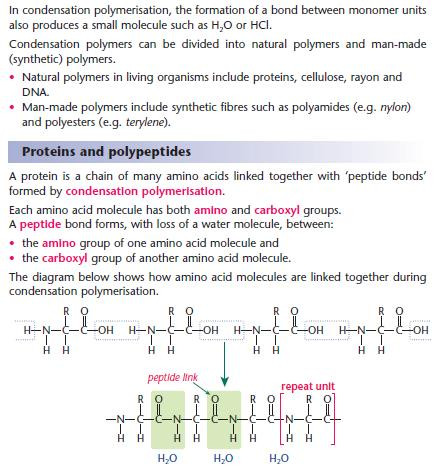
- In the repeat unit In the repeat unit above shown the R group may differ.
Polyamides
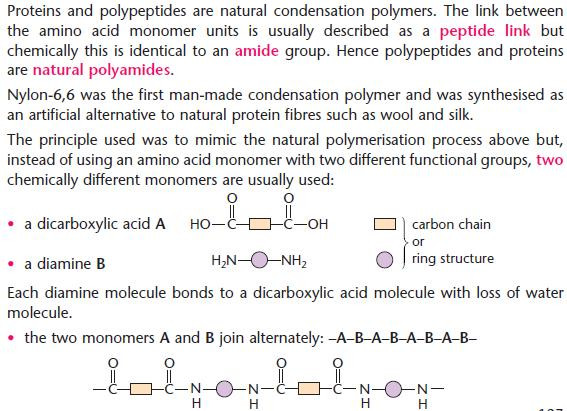
- Nylon, proteins and polypeptides are all polyamides.
- Compare the use of two monomers in the production of synthetic nylon-6,6 with the natural condensation
- polymerisation using amino acids only.
- Many different polyamides can be made using different carbon chains or rings which bridge the double
- functional group.
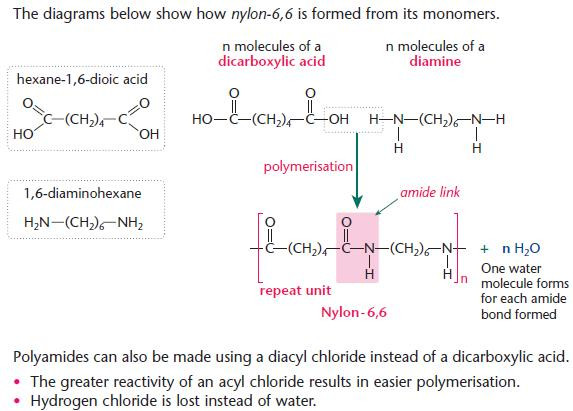
- Nylon-6,6 gets its name from the number of carbon atoms in each monomer (diamine first).
- The diamine has 6 carbon atoms.
- The dicarboxylic acid has 6 carbon atoms. Hence: nylon-6,6.
- The diacyl chloride for preparing nylon-6,6 would be ClOC(CH2)4COCl.
Polyesters
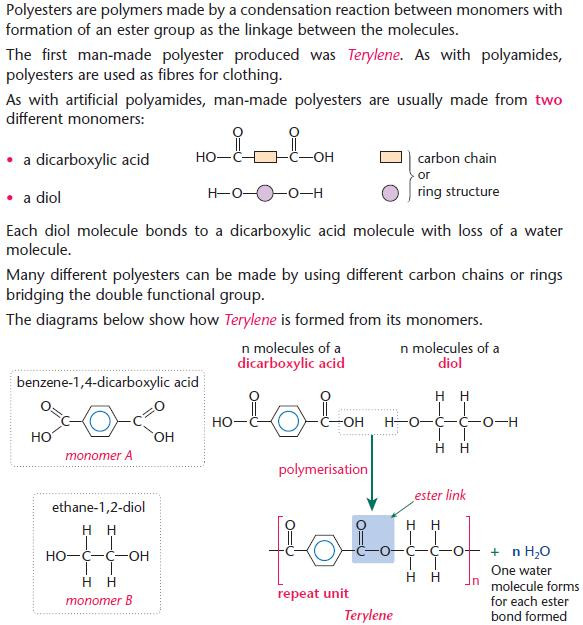
- Learn the principle behind condensation polymerisation.
- In exams, you may be required to predict structures from unfamiliar monomers. However, the principle is the same.
- This is the same basic principle as forpolyamides.
Disposal of polymers
Problems with addition polymers:
Addition polymers are non-polar and chemically inert.
This creates potential environmental problems during disposal of polymers.
Disposal by landfill causes long-term problems.
- Addition polymers are non-biodegradable and take many years to break down.
Disposal by burning can produce toxic fumes.
- Depolymerisation produces poisonous monomers.
- Disposal of poly(chloroethene) (pvc) by incineration can lead to the formation of very toxic dioxins if the temperature is too low.
Condensation polymers
Condensation polymers are polar.
- Condensation polymers are broken down naturally by hydrolysis into their monomer units. They are therefore biodegradable and easier to dispose off than addition polymers.