Reactions of Arenes
After studying this section you should be able to:
- describe electrophilic substitution of arenes: nitration, halogenation, alkylation, acylation and sulphonation
- describe the mechanism of electrophilic substitution in arenes
- understand the importance of reactions of arenes in the synthesis of commercially important materials
Many electrophiles react with alkenes by addition. However, electrophiles react with arenes by substitution, replacing a hydrogen atom on the ring. The difference in behaviour results from the high stability of the delocalised π-bonds in benzene compared with the localised π-bonds in alkenes.

In this section, reactions of benzene are discussed to illustrate electrophilic substitution reactions of arenes.
Nitration of arenes
The nitration of arenes produces aromatic nitro compounds, important for the synthesis of many important products including explosives and dyes
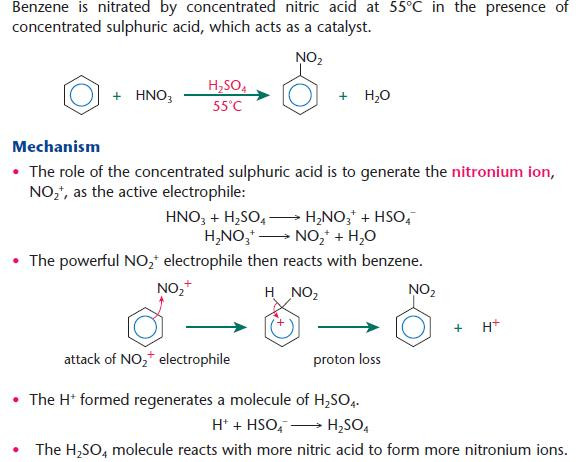

- Although some heat is required (55°C), too much may give further nitration of the benzene ring forming 1,3-dinitrobenzene.
- The nitronium ion is also called a nitryl cation.
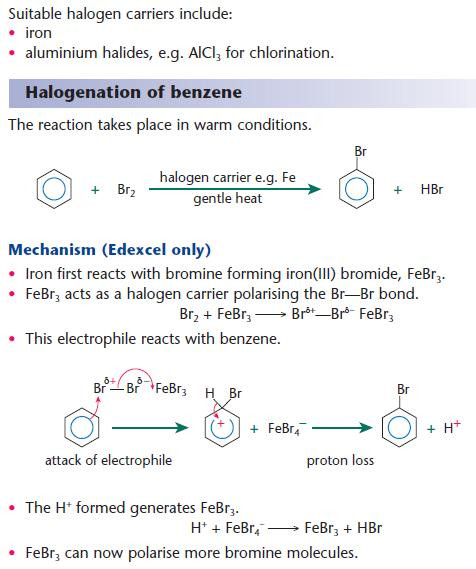
Halogenation of arenes
Arenes react with halogens in the presence of a halogen carrier, which acts as a catalyst.


Alkylation of arenes
Alkylation reactions of arenes are commonly known as Friedel–Crafts reactions.
These are very important reactions industrially as they provide a means of introducing an alkyl group onto the benzene ring.

- This is a similar principle to the halogenation of the benzene ring.
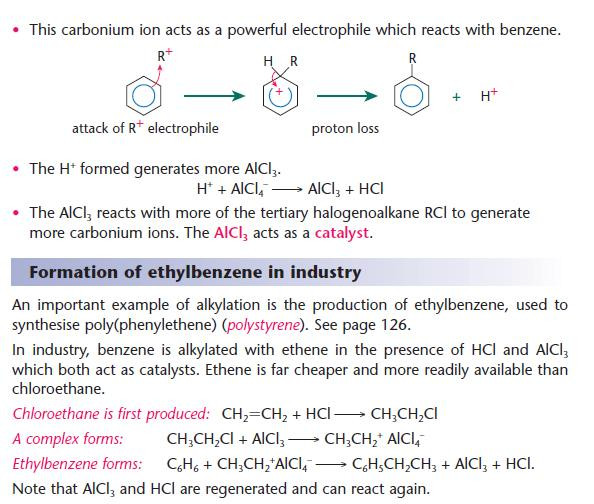
- By using different halogenoalkanes, different alkyl groups can be substituted onto the benzene ring.
- The reaction has been modified to make use of available starting materials.
- Benzene and ethene are produced in large quantities in refineries.
- Chloroethane is not readily available.
Sulphonation of arenes
Sulphonation of arenes produces sulphonic acids. Sodium salts of sulphonic acids are used for detergents and fabric conditioners.

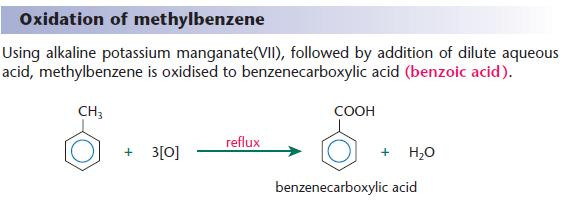
Oxidation of substituted arenes
The side chains of substituted arenes can be readily oxidised by prolonged reflux with a strong oxidising agent.
PROGRESS CHECK 2

