Atoms and Isotopes
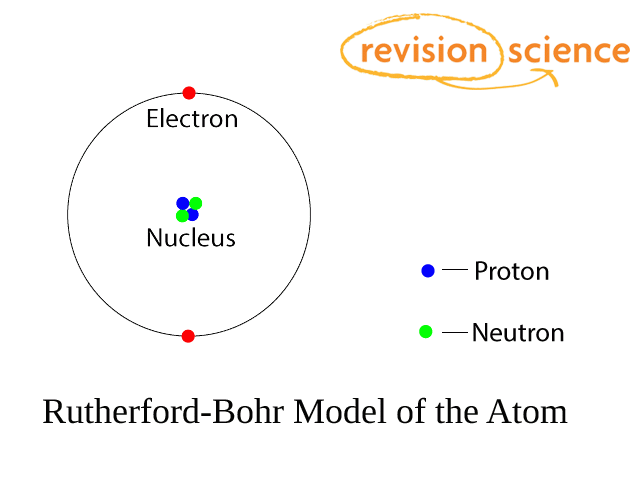
Atoms
Atoms have a small central nucleus made up of protons and neutrons around which there are electrons.
Image
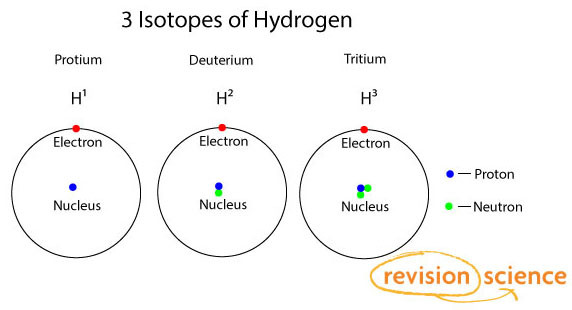
Isotopes
Atoms of the same element always have the same number of protons.
Isotopes are atoms of the same element but with different number of neutrons.
This gives rise to different mass numbers.
Relative abundance is the amount of each isotope as the percentage for that element occurring on the Earth.
Image