Hydrogen Bonding
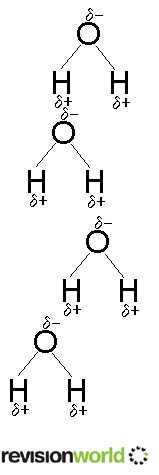
This diagram is only an approximation. A better one will be added as soon as possible.
Hydrogen bonding is a special case of permanent dipole-permanent dipole bonding. It is important to be clear that although it is called "hydrogen-bonding" it really is an intermolecular force. It is alsovital that you refer to the hydrogen bonding as being between molecules and not within them.
It exists where one of the most electronegative elements (fluorine, oxygen or nitrogen) is bonded to hydrogen.
Hydrogen bonding causes stronger intermolecular forces than would otherwise be predicted.
This increases the boiling point of substances such as water.
To investigate the power of hydrogen bonding, look at the boiling points of the group VI hydrides.
There is a steady increase down the group from H2S downwards but water (H2O) has a much higher boiling point despite being higher in the group.
It also explains why water is one of the few substances for which the solid is less dense than the liquid. On freezing, the hydrogen bonding organises the molecules into a fairly open structure.
