Alkanes
After studying this section you should be able to:
- understand why different alkanes have different melting and boiling
- understand that combustion of alkanes provides useful energy
- describe the substitution reactions of alkanes with halogens
Alkanes
Alkanes are generally unreactive. Alkanes contain only C–H and C–C bonds, which are relatively strong and difficult to break. The similar electronegativities of carbon and hydrogen give molecules which are non-polar. Alkanes are the typical ‘oils’ used in many non-polar solvents and they do not mix with water.
Melting points and boiling points of alkanes
Boiling points increase with increasing chain length because of the increasing strength of the intermolecular forces. This allows the alkane mixture in crude oil to be separated by fractional distillation.

The same principle can be applied to any homologous series.
For hydrocarbons of similar molecular mass, branched alkanes have lower boiling points because there are fewer interactions possible between molecules:
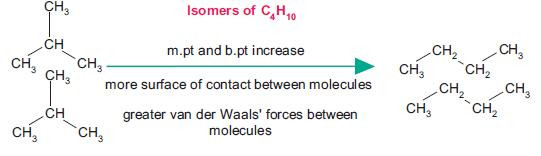
The surface contact between molecules is important because it allows interaction between electrons. Remember that van der Waals’ forces result from fluctuations in electron density
Combustion of alkanes
Combustion with oxygen is the most important reaction of alkanes giving their immediate use as fuels:
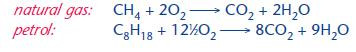
The burning of fossil fuels such as alkanes provides society with many environmental problems, some of which are listed below.
- Petroleum fractions contain sulphur impurities. Unless removed before combustion, sulphur oxides are formed from which acid rain (H2SO3 and H2SO4) is produced.
- Toxic gases such as carbon monoxide, nitrogen oxides and unburnt hydrocarbons are present in car emissions. The development of catalytic converters has helped to remove these polluting gases.
- Excessive combustion of fossil fuels increases carbon dioxide emissions contributing to the ‘Greenhouse Effect’.
Although generally unreactive, alkanes do react with oxygen and the halogens.
Remember that reactions with oxygen produceoxides.
Environmental issues are often tested in exams.
Substitution of alkanes with halogens
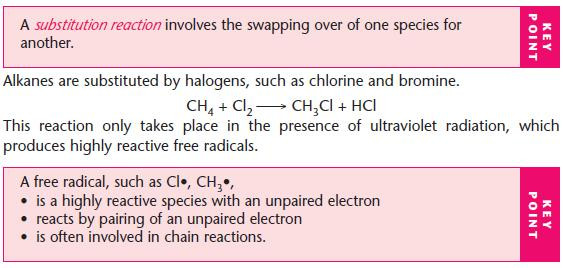
Mechanism of free-radical substitution (not EDEXCEL)
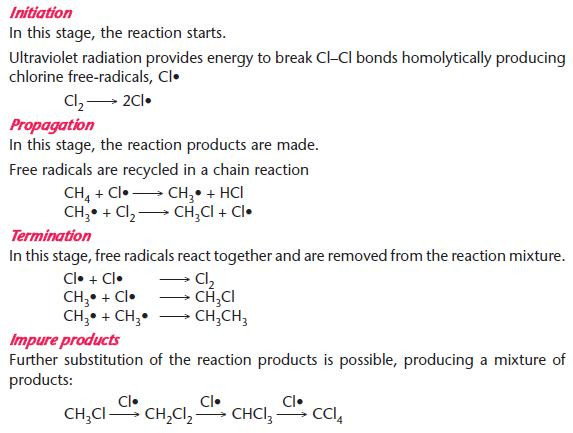
Make sure that you learn this mechanism – these are easy marks in exams if you do!
The chlorine free-radicals catalyse the reaction. See also the breakdown of ozone.
This is normally answered poorly in exams!
Progress check

