1st Law of Thermodynamics
Energy can be neither created nor destroyed (conservation of energy)
Thus power generation processes and energy sources actually involve conversion of energy from one form to another, rather than creation of energy from nothing
ΔQ = ΔU + ΔW
ΔU: Change in internal energy of the system
ΔQ: Heat transferred into/out of the system
ΔW: Work done by/on the system
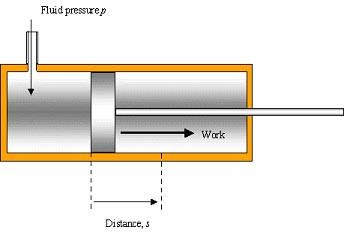
Cylinder has area, A. A fluid is admitted at constant pressure, p
p = F/A & Wd = fd … rearrange:
F = pA → Wd = pAd (Ad = volume, V)
→ Wd = pV or ΔWd = pΔV
pV = nRT (Ideal Gas Law)
Boyle’s Law: pV = constant
- Temperature remains constant (isothermal)
- pV = constant and p1V1 = p2V2
- ΔU = 0 because the internal energy is dependent on temperature, which does not change
- ΔQ = ΔW. If the gas expands to do work ΔW, & amount of heat ΔQ must be supplied
- compression or expansion produces the same graph
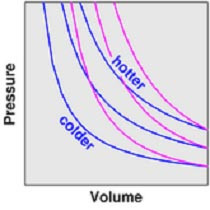
Adiabatic: no heat flow (ΔQ=0) into or out of a system
For a change in pressure or volume in a system, the temperature loss can be calculated:
p1V1/T1 = p2V2/T2
At high p, low V: adiabatic = value expected for isothermal at high T
At low p, high V: adiabatic cuts isothermal at low T
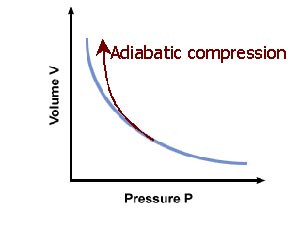
Equation for adiabatic line:
pVγ = k
γ = Cp/Cv
k= constant
Isovolumetric: p1T1 = p2T2
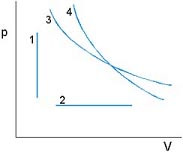
Isobaric: V1T1 = V2T2