Polymers
Polymers consist of long chain molecules; natural rubber is a polymer whose molecules have no ordering. Such polymers are called amorphous. Perspex is a synthetic polymer whose structure is also amorphous. Nylon and polythene have both crystalline and amorphous regions, their structure is described as semicrystalline.
The diagram below shows how the chains in a semi-crystalline polymer are folded; the straight rows represent crystalline regions and the bends are amorphous.
Amorphous describes any structure that is irregular, with no ordering. Glass has an amorphous structure.
Both nylon and polythene soften on heating because the bonds between the long chain molecules are weak; they are called thermoplastics. Because of this property they can be used to manufacture articles by moulding.
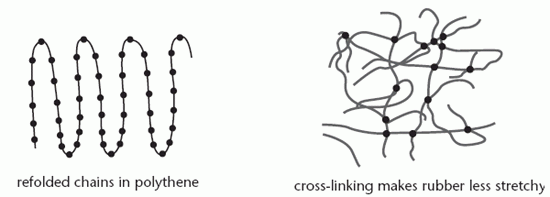
The rigidity of a polymer is increased by cross-linking, the formation of strong bonds between the molecules by introducing other substances. The vulcanised rubber used in car tyres contains sulphur which forms bonds between chains, making it less elastic. Adding more sulphur makes the rubber more rigid. As more cross-linking is employed, the polymer becomes a thermoset, one which hardens with increasing temperature.
The rubber used in the manufacture of car tyres has a low sulphur content, about 5%. Hard rubbers contain up to 50% sulphur.
Thermosetting polymers are used in the manufacture of electrical fittings such as lamp holders, sockets and kitchen worktops. Because they cannot be softened by heating, any moulding has to be done as the polymer is manufactured.
Bakelite and melamine are thermosets commonly used for household objects.
