Constituents of the Atom
For most elements, a sample contains a mixture of different versions.
These have the same number of protons, and electrons, but a different number of neutrons.
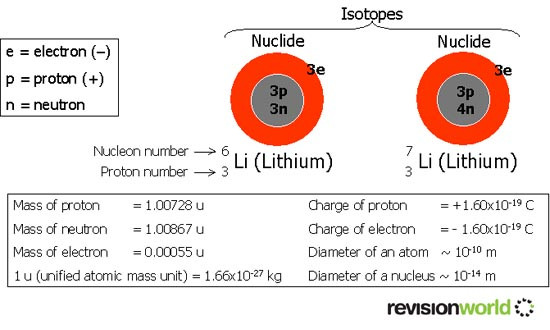
Nuclide - This is a particular version of an atom. The previous example shows a simple model of the two naturally occurring nuclides of Lithium, along with the symbols used.
Nucleon Number A - This number is made up of the total number of protons and neutrons in the nucleus, also called nucleons. Once called the mass number.
Proton Number Z - This is the number of protons in the nucleus, and also the number of electrons in a neutral atom. Once called the atomic number.
Isotopes - These are atoms with the same proton number, but different nucleon numbers. They have the same electron arrangement and, therefore, the same chemical properties
