Useful Products from Rocks
This section covers
- VIDEO: Useful products from Metal Ores and Rocks
- Extracting Iron from a Blast Furnace
- Extraction of Aluminium
- Purification of Copper
- Limestone
- Cement, Mortar, Concrete, Glass
- Corrosion
Rocks from the Earth contain many useful metals. Most metals are combined with other elements in materials called ores and have to be extracted using various methods. How each metal is extracted depends on how reactive it is.
Gold is a very unreactive metal and is found as a pure metal, because of this it has been in use for many thousands of years despite it being a very rare metal.
Iron and copper are more reactive than gold but less reactive than carbon, these can be extracted from their ores by simply heating with coke. These have been known for several thousand years
Aluminium is the most common metal in the Earth’s crust, however, was only discovered 200 years ago because it is a relatively reactive metal which is hard to extract from its ore. When Napoleon held a banquet, the moderately important guests were given gold plates, the really special people were given aluminium ones!
This video explains what useful products can come from Metal Ores and Rocks
Extraction of Iron Ore from a Blast Furnace
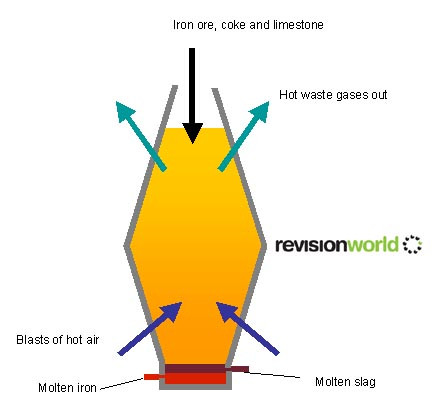
A metal such as iron, which is less reactive than carbon, can be extracted from its ore using carbon
Reactions in the blast furnace:
- C + O2 > CO2
- The coke burns, to form carbon dioxide and to produce heat
- CO2 + C > 2CO
- The carbon dioxide reacts with more hot coke to produce carbon monoxide gas.
- 3CO + Fe2O3 > 2Fe + 3CO2
- The carbon monoxide removes the oxygen from the iron ore this is called reduction.
- The main impurity in the iron ore is silica this reacts with the limestone to produce slag (calcium silicate)

Copper can be extracted from its ore by reduction with carbon, however, this is only 98% pure.
Copper can be purified by electrolysis using a positive electrode made of the impure copper and a negative electrode of pure copper in a solution containing copper ions.
When the current is switched on copper ions in solution are attracted to the negative and electrode and deposited there.
Copper atoms in the impure block lose electrons and become positive ions and go into the solution, replacing those which were deposited at the negative electrode.
Eventually the impure block disappears leaving behind the impurities and the pure block becomes larger.
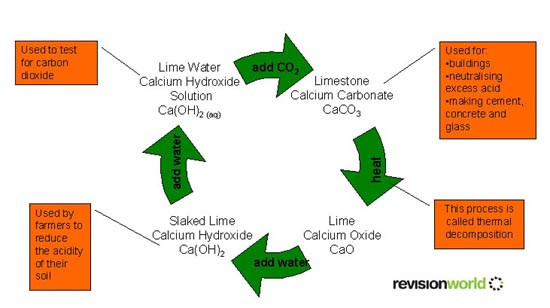
This video from the BBC explains about Limestone and the Chemistry behind this
Cement, Mortar, Concrete, Glass
They are all "to do with limestone" but you nedd to be more specific.
Cement: Limestone is mixed with clay, heated strongly and then crushed.
Mortar: Sand and cement are mixed together with water. It sets hard. It is usually mortar that holds the bricks together in a wall.
Lime Mortar: An older version of mortar that uses slaked lime with sand and water. It is often used to repair old buildings (where it replaces the lime martar that was originaly in place).
Concrete: Sand, cement and small stones (often called aggregate) are mixed together with water. It sets hard. Sometimes the concrete is poured over steel rods. This is known as reinforced concrete.
Glass: There are many ways to make glass but here we need to know about heating a mixture of limestone, sand and sodium carbonate.
This video explains more into how Limestone is used for industry
Iron rusts in the presence of oxygen and water.
Barriers such as paint or grease can be used to prevent corrosion.
Zinc blocks can be attached to iron objects. As zinc is more reactive than iron it will corrode preferentially thus preventing the iron from corroding. This is called sacrificial protection.
Aluminium is a very reactive metal but it can be used without protection against corrosion. This is because it has a thin layer of oxide which sticks very firmly to the aluminium and protects it against further corrosion.
