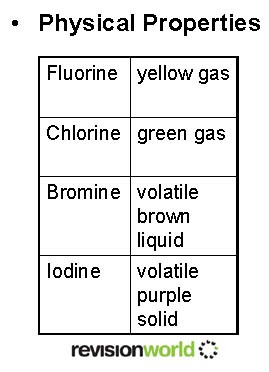
Group 7 – The Halogens
Image
Chemical Properties
- All halogens form diatomic molecules, that is they go around in pairs e.g. I2
- The halogens are reactive elements, with reactivity decreasing down the group.
- The larger atoms are less reactive as it is harder for them to capture an electron.
- Halogen compounds are called halides. Chlorine forms chlorides, bromine forms bromide etc.
