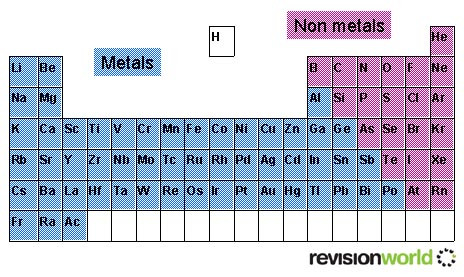
The Periodic Table
This modern periodic table lists elements according to their atomic number, if they were arranged according to atomic mass potassium and argon would be the wrong way round.
Elements having the same number of electrons in their outermost shell are placed in vertical columns called groups. They have similar chemical properties.
From left to right, across each horizontal row (period) of the periodic table, a particular energy level is gradually filled up with electrons; in the next period, the next energy level is filled with electrons.
This video explains about the Atomic Number
But how did we get to this state of knowledge?
Development of the Periodic Table
The list of elements was originally conceived in order of “atomic weight”.
Newlands – Law of Octaves. Every eighth element seemed to be similar, rather like every eighth note in music. One example he used was Li, Na, K.
Mendeleev – He made the table two-dimensional rather than a list. He arranged the elements in order of atomic weight but had the Newlands Octave elements underneath each other. The vertical columns are called groups and the horizontal rows are called periods. The term periodic refers to the fact that the properties swing to and fro like a pendulum.
Some elements had not been discovered at the time of Mendeleev writing up his table so he left gaps to indicate where elements ought to be. This was brave because a theory with gaps in it looks a bit silly unless you can explain why the gaps are there. Later chemists discovered elements to fit in the right places and they were amazed to find that the properties were very much like those Mendeleev had predicted.
When the noble gases were discovered, argon was a problem because if the elements are arranged in order of atomic weight, argon and potassium should swap places. Fortunately, more detailed theories about the structure of the atom were being proposed. If these theories (using protons, neutrons and electrons) were right, it would be more sensible to arrange the elements in order of atomic number (number of protons). If this is done, the problem of argon and potassium is solved. We now know that the group number of an element tells us the number of electrons it has in its outside shell.
This video explains how the Periodic table was constructed
