Particles and Heat Transfer
Heat Transfer
Heat can be transferred by conduction, convection and radiation. It is only transferred from hotter things to cooler things.
Heat is transferred in order to equalise the temperatures of the object and its environment
Examples:
- A cup of coffee will cool down because it is giving out heat energy into the surroundings
- A cold drinks can (taken out of the fridge) will warm up because it is taking in heat energy from the surroundings
Conduction
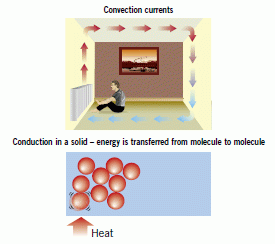
In a hot solid, particles vibrate more. They collide with the particles next to them and set them vibrating. The kinetic energy is transferred from particle to particle. Metals are the best conductors. Solids are better than liquids. Gases are very poor conductors. They are insulators.
Atoms in a substance are always vibrating.If the substance gets hotter, the atoms vibrate more. The heat energy is given to the atoms, which makes them move about faster
Every time they collide with another atom, the heat energy is transferred. This is how heat travels through a solid
The video below shows how heat transfer occurs through conduction
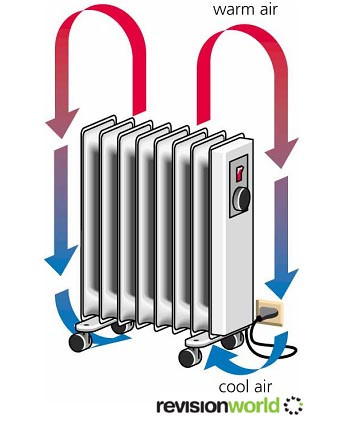
Convection
In a hot fluid (gas or liquid) the particles have more kinetic energy so they move more. They spread out and the fluid becomes less dense. The hot fluid rises above the denser cold fluid forming a convection current.
- Hot air rises in cold air
- Hot water rises in cold water
- This is called convection
When hot air rises, colder air has to move in to replace it. Convection cannot happen in solids, as the atoms aren't able to move around
The video below explains how heat transfer occurs by convection
Radiation
All objects emit and absorb infrared radiation. The higher the temperature the more they emit.
When objects absorb this energy their temperature increases.
Radiation will travel through a vacuum – it does not need a medium (material) to pass through:
- Dark and matt surfaces are good absorbers and emitters of infrared radiation.
- Light and shiny surfaces are poor absorbers and emitters of infrared radiation.
- Light and shiny surfaces are good reflectors of infrared radiation.
The video below explains about how heat transfer occurs by Radiation
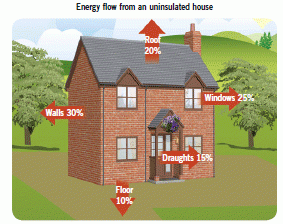
Insulation
When we insulate our homes we reduce the heat lost, we use less fuel and it costs less. Still air is a good insulator, so materials with air trapped in them are often used:
- In cavity walls the air gap between the walls stops conduction.
- In cavity wall insulation the cavity is filled with foam or mineral wool.
- Loft insulation using layers of fibreglass or mineral wool
- Reflective foil on walls reflects infrared radiation.
- Draught-proofing stops hot air leaving and cold air entering the house.
All these improvements cost money to buy and install, but they save money on fuel costs. You can work out the payback time which is the time it takes before the money spent on improvements is balanced by the fuel savings, and you begin to save money:
Payback time (in years) = cost of insulation ÷ cost of fuel saved each year
If the price of the fuel increases, the payback time will be less.
Conductors and insulators
This video opens by asking the question 'How many wires go into a kettle?' before moving to a cut-away diagram of a three-core mains cable with an animation of electrons moving in the live and neutral wires. The ability of free/delocalised electrons to conduct electricity is then discussed.
